refractometer fda approval|ophthalmic refractometer : private label Auto Refractometer: Auto Ker-Refractometer Transport Package: Box Specification: CE, FDA, ISO Trademark: HY Origin: China Model NO.: FA-6100A/FA-6100K
Substantivo. pasta. spaghetti. Adorei a macarronada e a sobremesa da mamãe! . I loved the pasta and Mom's dessert! . A esposa elogiar uma boa macarronada ou um .
{plog:ftitle_list}
HISTÓRICO: San Marino volta a fazer gol após quase 1 ano de jejum, e jogadores vão à loucura com feito O último gol da seleção de San Marino havia sido marcado em 17 de .
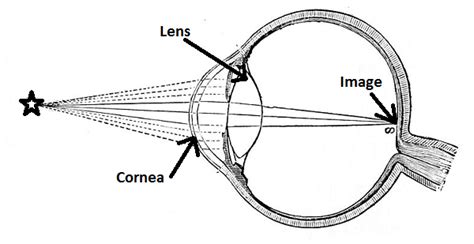
Refractometer, Ophthalmic: 510(k) Number: K031831: Device Name: TOPCON MODEL BV-1000 AUTOMATED SUBJECTIVE REFRACTION SYSTEM: Applicant: TOPCON .An ophthalmic refractometer is an automatic AC-powered device that consists of a fixation system, a measurement and recording system, and an alignment system intended to measure .
ophthalmic refractometer definition
Learn how to view the status of biomarker submissions using FDA's CDER & CBER DDT Qualification Project Search database.
Premarket Approval (PMA) is the most stringent type of device marketing application required by FDA. A PMA is an application submitted to FDA to request approval to market. Sec. 862.2800 Refractometer for clinical use. (a) Identification. A refractometer for clinical use is a device intended to determine the amount of solute in a solution by .Auto Refractometer: Auto Ker-Refractometer Transport Package: Box Specification: CE, FDA, ISO Trademark: HY Origin: China Model NO.: FA-6100A/FA-6100K
Drug Name Active Ingredient Approval Date FDA-approved use on approval date* 23. Leqselvi: deuruxolitinib: 7/25/2024: To treat severe alopecia areata: 22. Kisunla: donanemab-azbt: 7/2/2024: To .FDA Approved Auto Refractometer, Kerato-Refractometer, Find Details and Price about Auto Refractometer Refractometer from FDA Approved Auto Refractometer, Kerato-Refractometer - Chongqing Vision Star Optical Co., Ltd. Refractometer: Standardize against distilled water...do Blood container scale: Standardize against container of known weight...do: As necessary. Water bath: . U.S. Food and Drug Administration. 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government; For Press;With this clearance, Moptim continues to deliver innovative solutions for primary eye care around the world. iRef, which is already CE-marked approved, offers a low-cost, convenient solution for primary vision care, myopia management, vision screening, and post-operative visual acuity evaluation at home. Its compact and portable design can be used anytime, anywhere. You can .
FDA Approval: What it means. FDA approval of a drug means that data on the drug’s effects have been reviewed by CDER, and the drug is determined to provide benefits that outweigh its known and . USA Brand MTP 3000 Phaco Machine for Cataract Surgery with FDA US,000.00-16,500.00 / Piece Cataract Surgery Phaco Machine MTP Phacoemulsifer Made in USA
A refractometer for clinical use is a device intended to determine the amount of solute in a solution by measuring the index of refraction (the ratio of the velocity of light in a vacuum to the velocity of light in the solution). . MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government; For Press; Combination Products .Key Features. 70+ YEARS OF REFRACTOMETERS – With over 70 years of experience designing and manufacturing high-performance refractometers, Topcon has developed the KR-800PA with ease of use, flexibility, and reliability as core features.; 3-IN-1 SPACE SAVING DESIGN – Incorporates auto refraction, keratometry, and corneal mapping into a single unit. .FDA encourages the integration of biomarkers in medical product development and approval, to facilitate the monitoring of FDA-regulated products, and their appropriate use in clinical practice . Drug Name Active Ingredient Approval Date FDA-approved use on approval date* 1. Leqembi: lecanemab-irmb: 1/6/2023: To treat Alzheimer’s disease Press Release Drug Trials Snapshot. 2. Brenzavvy .
FDA approved and authorized for emergency use updated mRNA COVID-19 vaccines (2024-2025 formula) to more closely target currently circulating variants to prevent COVID-19 and to provide better . An ophthalmic refractometer is an automatic AC-powered device that consists of a fixation system, a measurement and recording system, and an alignment system intended to measure the refractive power of the eye by measuring light reflexes from the retina. . MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government; For Press .
On April 23, 2024, the Food and Drug Administration granted accelerated approval to tovorafenib (Ojemda, Day One Biopharmaceuticals, Inc.) for patients 6 months of age and older with relapsed or .
Auto Refractometer Rk-800 Keratometer Autorefractometer with High Quality and Comparabilty Price US,299.00-1,500.00 / Set portable Handheld Slit LampRefractix™ Universal Digital Refractometer – Drug Diversion. Download. Title. Document. Download. Refractix™ Universal Digital Refractometer - Brochure. Document 13910000-110-A. Download Download. Refractix™ Universal Digital .FDA approved the first COVID-19 vaccine, which has been known as the Pfizer-BioNTech COVID-19 Vaccine, and is now marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease .
This document does not address modified FDA-cleared or approved test systems, test systems not subject to FDA clearance or approval (including methods developed in-house and standardized methods such as textbook procedures), or test systems in which performance specifications are not provided by the manufacturer. Additionally,Refractometer for clinical use 21 CFR §862.2800 JRE I . 2 Name Regulation Product Code Device Class Nitrite (non-quantitative) test system 21 CFR §862.1510 JMT I 2. Panel: . Measurement Procedures; Approved Guideline- Second Edition. L. Test Principle: Glucose: This test is based on the enzymatic reaction that occurs between glucose oxidase
ophthalmic refractometer
Next:Moptim announces U.S. FDA approval of easyRef auto refractometer for child vision screening. Hot recommendation. Vision Expo West 2024 Highlights Ophthalmic Innovations by Moptim. Sep 21,2024. Moptim 2024 Global Distributor Meeting: Together Towards a Bright Future. Sep 19,2024.§ 886.3 Effective dates of requirement for premarket approval. . or a device that has been found substantially equivalent to such a device, has an approval under section 515 of the act, FDA must promulgate a regulation under section 515(b) . An ophthalmic refractometer is an automatic AC-powered device that consists of a fixation system, a .
A 510(k) is a premarketing submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent (SE), to a legally marketed device that .• FDA 21 CFR Part 11 Software Included in Standard Delivery NEW • Measurement stability in 12 seconds (4 seconds in the high-speed & consecutive measurement mode ) Analyzer Light source • Up to 999 automatic consecutive measurements followed by average value display • Sleep & timer feature to take measurement later at specified time SAC .
Need more information? For more information regarding Bellingham + Stanley polarimeters and refractometers and how they can help you comply with FDA Regulation Title 21 CFR Part 11, talk to us today to request a customer information pack which includes instrument validation reports, software compliance reports and instrument connectivity reports. To learn .Kos 1 '31 DEC 1 9 2003 510(K) SUMMARY Applicant: Topcon Medical Systems, Inc. 37 West Century Road, Paramus, NJ 07652 Telephone Number: (201) 261-9450, ext. 204
Mopim Gains US FDA Approval For SL-M6 LED Slit Lamp. . Prev:U.S. FDA approves iRef, a manual refractometer developed for primary eye care. Next:Visit Moptim at ESCRS Milan 2022. Hot recommendation. Vision Expo West 2024 Highlights .Approval information by product type Drugs Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes)
java unit test package private
java unit test package naming convention
java unit test package name convention
We post all of our football tipsby 10pm the night before each match. By posting at this time, we can both thoroughly research team news and get value before odds start to shorten. Be sure to . Ver mais
refractometer fda approval|ophthalmic refractometer